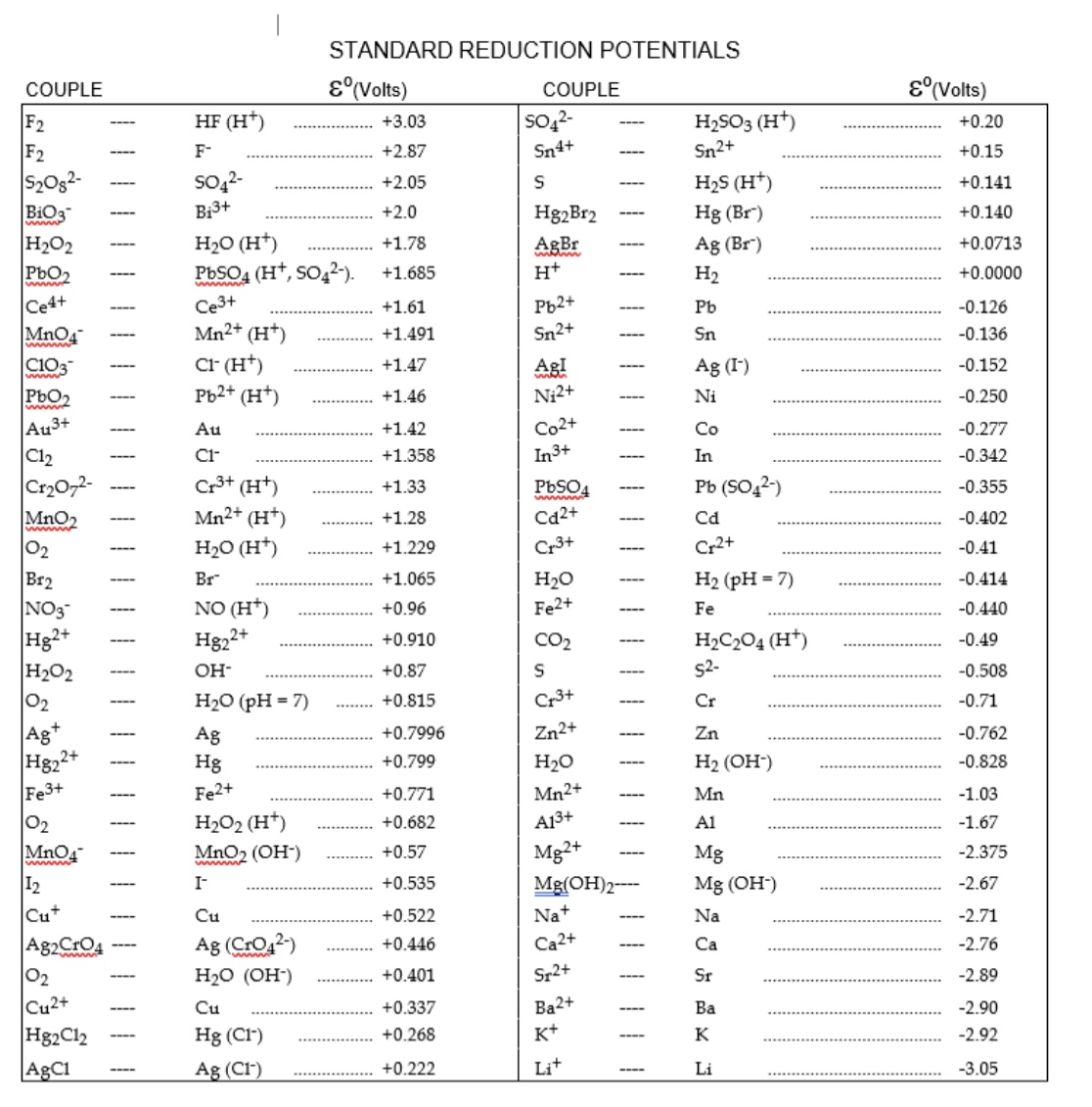
Standard Cell Potentials . revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. to do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of calculating cell potential for redox.
from
to do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. See examples of calculating cell potential for redox. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions.
Standard Cell Potentials See examples of calculating cell potential for redox. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. See examples of calculating cell potential for redox. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. to do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions.
From
Standard Cell Potentials learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. See examples of calculating cell potential for redox. to do this, chemists use the standard cell potential (e°. Standard Cell Potentials.
From
Standard Cell Potentials to do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by. Standard Cell Potentials.
From
Standard Cell Potentials we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the cell potential is the way in which we can measure how much. Standard Cell Potentials.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Cell Potentials to do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. See examples of calculating cell potential for redox. learn the definition and formula of standard cell potential, and. Standard Cell Potentials.
From
Standard Cell Potentials learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. we can calculate the standard potential for any electrochemical cell from the standard potentials of. Standard Cell Potentials.
From www.youtube.com
Calculating Standard Cell Potential YouTube Standard Cell Potentials the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. learn the definition and formula of standard cell potential, and. Standard Cell Potentials.
From www.youtube.com
calculation of half cell potential electrochemistry 2 class 12 Standard Cell Potentials revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. to do this, chemists use the standard cell potential (e° cell), defined as the potential. Standard Cell Potentials.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Standard Cell Potentials See examples of calculating cell potential for redox. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. to do this, chemists use the standard cell potential (e° cell), defined as. Standard Cell Potentials.
From
Standard Cell Potentials See examples of calculating cell potential for redox. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. the cell potential is the. Standard Cell Potentials.
From www.youtube.com
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials Standard Cell Potentials the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. See examples of calculating cell potential for redox. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. revision notes on 5.3.3 standard electrode & cell potentials for. Standard Cell Potentials.
From quizdbbarnstorms.z21.web.core.windows.net
What Is Standard Cell Potential Standard Cell Potentials See examples of calculating cell potential for redox. to do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. we can calculate the. Standard Cell Potentials.
From exoeelsdz.blob.core.windows.net
Standard Electrode Potentials Chemguide at Richard Reddish blog Standard Cell Potentials we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts. Standard Cell Potentials.
From
Standard Cell Potentials learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of calculating cell potential for redox. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. to do this, chemists use the standard cell potential (e° cell), defined as. Standard Cell Potentials.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Cell Potentials revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. we can calculate the standard potential for any electrochemical cell. Standard Cell Potentials.
From 2012books.lardbucket.org
Standard Potentials Standard Cell Potentials the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. See examples of calculating cell potential for redox. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. learn the. Standard Cell Potentials.
From
Standard Cell Potentials the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. See examples of calculating cell potential for redox. to do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under. learn the definition and formula of standard. Standard Cell Potentials.
From
Standard Cell Potentials See examples of calculating cell potential for redox. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the cell potential is the way in which we can measure how much voltage exists between the two half cells of a. learn the. Standard Cell Potentials.
From
Standard Cell Potentials revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. the cell potential is the way in which we can measure how much. Standard Cell Potentials.